
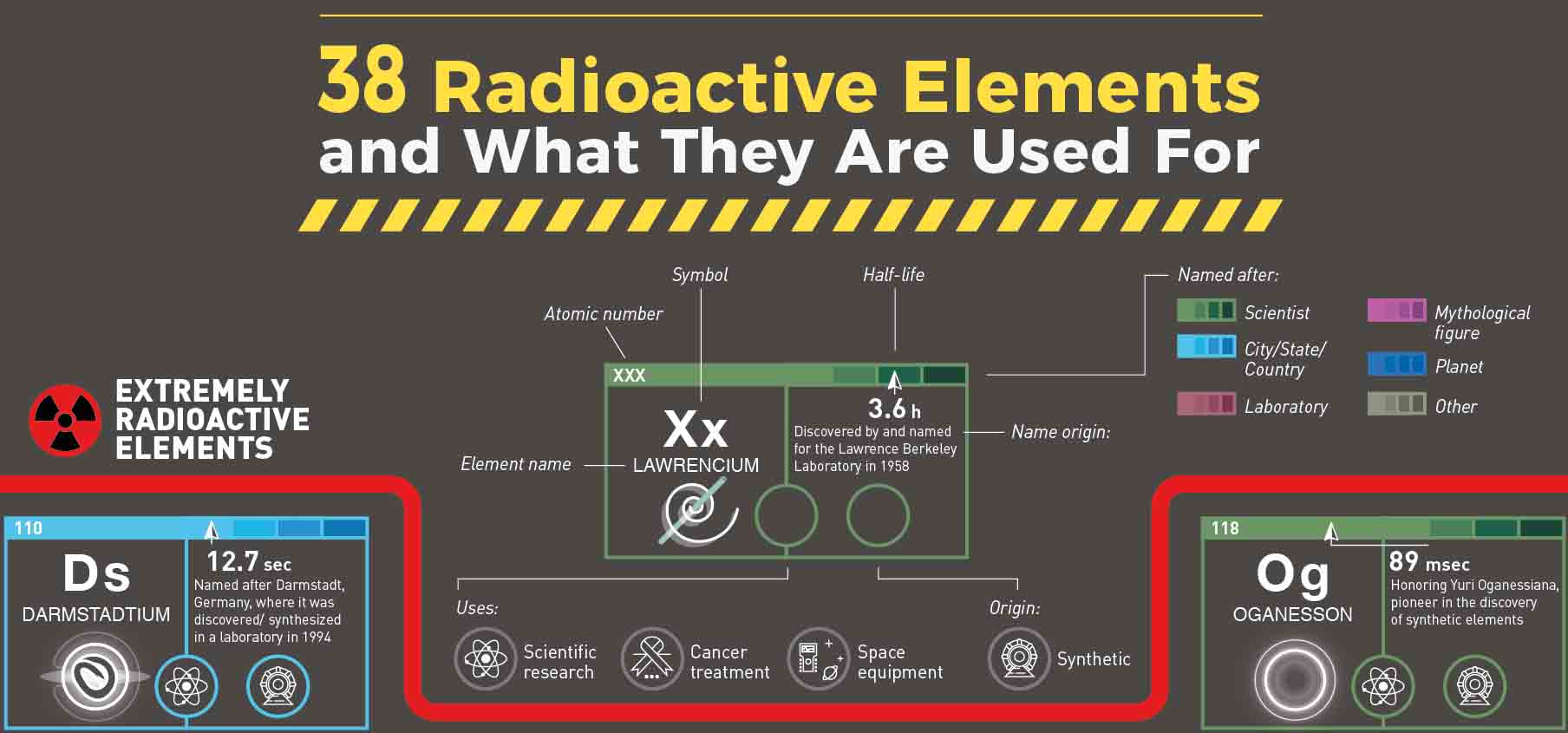
As a radioisotope tries to stabilize, it may transform into a new element in a process called transmutation. Elements with atomic numbers of 83 and less, have isotopes (stable nucleus) and most have at least one radioisotope (unstable nucleus). Radioactive material is a class of chemicals where the nucleus of the atom is unstable. Radioactive isotopes are often called radioisotopes.Īll elements with atomic numbers greater than 83 are radioisotopes meaning that these elements have unstable nuclei and are radioactive. The four common radioactive elements are Uranium, Radium, Polonium, and Thorium. Radioactive elements do not have standard atomic weights but many versions of the Periodic Tables include the mass number of the most stable isotopes, usually in square brackets. So any elements above lead are unstable and can decay in some form or another, once a decay happens. As the unstable nucleus changes, it gives off radiation and is said to be radioactive. Lead has the highest atomic number for stable elements. In an unstable atom, the nucleus changes its configuration by giving off matter of energy to get to a balanced state. However, each additional neutron may upset the balance and cause the atom to become unstable. Even if an atom has an additional neutron or two it may remain stable. Stable atoms have a binding energy that is strong enough to hold the protons and neutrons together. In most cases, elements like to have roughly the same number of protons and neutrons because this makes them the most stable. The atomic mass of naturally occurring but radioactive potassium-40 is measured to be 39.964008 amu. The nucleus is held together by something called the binding energy. A precise measurement of the masses of atoms involved in radioactive decay is equivalent to direct measurement of the energy release in the decay process. Because the like charges of the protons repel each other, these forces are always trying to push the atomâs nucleus apart.
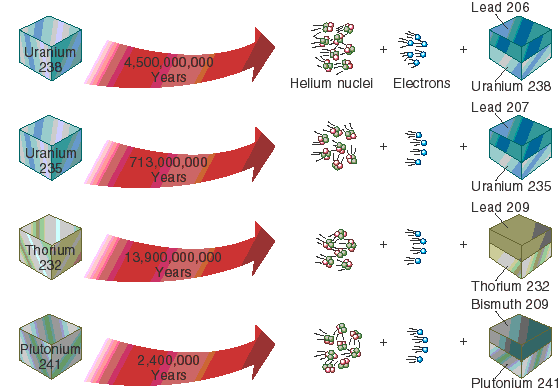
What is a radioisotope?Īs discussed in more detail elsewhere, isotopes are variants of an element that, while all having the same number of protons, have differing numbers of neutrons. Radioactivity is the release of energy or ejection of matter that results from changes in the nucleus of an atom.

The unstable nucleus will emit energy in some form and is said to be radioactive. Through Einstein’s equation, energy is equal to mass ( m) times velocity of light ( c) squared, or E mc2, the energy release ( Q) and the mass. Potassium-40 decays predominantly by -emission to calcium-40, having a measured mass 39.962589. Explain how a radioisotope differs from an isotope.Ītoms with unstable nuclei are constantly changing as a result of the imbalance of energy within the nucleus. The atomic mass of naturally occurring but radioactive potassium-40 is measured to be 39.964008 amu.Atoms that contain an unstable combination of neutrons and protons, or excess energy in their nucleus. Define radioactivity and explain how it is produced. Radioisotopes are radioactive isotopes of an element.After reading this section you will be able to do the following:


 0 kommentar(er)
0 kommentar(er)
